
Background
The Water Supplies Department (WSD) of Hong Kong has been providing drinking water in accordance with the World Health Organization (WHO) Guidelines for Drinking Water Quality, but since the incident of excessive lead in drinking water, the safety requirements of the Government, the practitioners and the public have increased significantly. After consulting experts, the WSD formulated the Plan for Enhancing Drinking Water Safety in Hong Kong on year 2017. In the past, the WSD placed more emphasis on testing the physical, chemical and bacteriological parameters of water samples at WSD's water supply points, but through this scheme, the material requirements and acceptance of newly installed drinking-water systems have been increased. The scheme consists of five sections, namely "Enhanced Drinking Water Standards and Water Quality Monitoring", "Enhanced Regulation of Internal Water Supply System", "Water Safety Plan", "Publicity and Public Education", and "Drinking Water Safety Regulatory System".
Among them, the strengthening of the regulation of internal water supply systems include the supervision of plumbing materials, inspection and acceptance requirements for the construction of new plumbing installations, training and regulation of licensed plumbers, and the regulatory framework and legislation for carrying out plumbing works. One of them, the construction inspection and acceptance regulations of new water pipe installations are based on the systematic flushing procedures and the qualified test results of 6-hour stagnation water samples, which are designed to reduce the heavy metals released from the materials of the new water supply system during use, including lead, nickel, chromium, cadmium, copper, antimony, these six kinds of heavy metals, and the sources of these heavy metals may be faucets, fittings, valves and water meters of the water supply system. The following will describe in detail the content, testing principles, procedures and standards of the relevant regulations, so that you can further understand this regulation.
The scope of the requirements and the acceptance criteria for new plumbing works in occupied buildings
The inspection and acceptance requirements for the construction of new plumbing installations are for newly built water supply systems. For new plumbing works in used buildings, systematic flushing in the new system is usually not feasible, so pre-treated plumbing fittings or low leachability fittings are required instead of systematic flushing.
Pre-treated plumbing fittings are treated as follows [1]:
- Soak the fitting in a container of water for five days
- The fitting should be fully opened to ensure that its internal surface is fully immersed in water
- The water used to soak the fittings should be changed once a day, and the fittings should be rinsed simply with water
- During the soaking process, the container should be covered to maintain good hygiene
Plumbing fittings that require pre-treatment are only suitable for fittings with copper alloy internal surfaces that come into contact with water. Because iron can corrode, soaking copper alloy fittings should be avoided if they contain iron parts.
Low leachate refers to the fittings tested by the "Extraction of Metal" test method specified in the AS/ NZS 4020:2005 standard in Australia/ New Zealand, where the maximum allowable metal concentrations (lead, nickel, chromium, cadmium) do not exceed the limits specified in the World Health Organization's Guidelines for Drinking Water Quality.

Details of Systematic Flushing Procedures for New Plumbing Works
For new plumbing projects across the country, the Water Supplies Department has developed a set of systematic flushing procedures, which are detailed below:
1. The principle of flushing
The copper alloy fittings of the water supply system have high levels of heavy metals, especially lead, and the effect of adding lead is to improve the machinability of the copper alloy, but the increase in lead content will increase the risk of lead release into the water.
In the production process of copper alloy fittings, there will be cutting and grinding processes, so that the heavy metals in the copper alloy are exposed. In the early stages of the use of the water supply system, more heavy metals are released from the water, but after flushing, the heavy metal levels will decrease very quickly. At the same time, an oxide protective layer will be formed on the surface of the copper alloy when flushed, reducing the release of lead.
Therefore, if a new water supply system can be pre-flushed and oxide film generated, the risk of heavy metals exceeding the upper limit will be reduced.
2. The process of systematic flushing
The contractor or licensed plumber is required to carry out three flushing cycles of the water supply system according to the following procedures, each cycle consists of the following steps 1 to 4 [1]:
- Rinse the internal drinking water supply system thoroughly from the drinking water faucet
- Allow the water to rest in the system for at least 3 hours
- Repeat step 1
- Allow the water to rest in the system overnight (at least 12 hours).
The start and end times need to be recorded during each flushing process. After the entire flushing cycle, the internal water supply is thoroughly flushed again from the drinking water faucet, which can further help reduce the release of heavy metals.
3. Testing Requirements
After systematically flushing the internal drinking water supply system, the drinking water was allowed to stand still for 6 hours before the sample was taken for water testing. The test included heavy metal content, chemical and physical parameters, and bacterial parameters of the water samples. If the test result of the metal parameters is unsatisfactory, the contractor or licensed plumber is required to conduct another systematic flushing cycle before testing [1].
1. Heavy Metal Testing Standards:
Test items | Acceptance criteria |
Lead (μg/L). | ≤ 10 |
Chromium (μg/L). | ≤ 50 |
Nickel (μg/L). | ≤ 70 |
Cadmium (μg/L). | ≤ 3 |
Copper (μg/L). | ≤ 2000 |
Antimony (μg/L). | ≤ 20 |
2. Chemical and Physical Testing Standards:
Test items | Acceptance criteria |
Turbidity (NTU). | ≤ 3.0 |
Chromaticity (HU). | ≤ 5 |
pH (at 25 °C). | ≥ 6.5 and ≤ 9.2 |
Free residual chlorine (mg/L). | >0 and ≤ 1.5 |
Electrical conductivity (at 25°C) (μS/cm). | ≤ 300 |
3. Bacteriological Test Standards:
Test items | Acceptance criteria |
Escherichia (cfu/100mL). | 0 |
4. Rinse data reference
A study from Australia [2] also showed a reduction in copper and lead levels after flushing. The study took 10 pieces of data from different parts of Australia, and the water samples were taken after being left in the water supply for nine hours and rinsed for two minutes. The data analyzed are as follows:
Copper (μg/L). |
| Lead (μg/L). | ||
After a 9-hour stay | Rinse for 2 min later | After a 9-hour stay | Rinse for 2 min later | |
670 | 230 | <1 | <1 | |
2040 | 280 | 3.0 | 1.9 | |
1510 | 1080 | 1.8 | 2.2 | |
140 | 20 | 2.0 | <1 | |
11 | 2.1 | 10.0 | 1.1 | |
1137 | 525 | 3.0 | 5.8 | |
362 | 188 | 5.6 | 4.6 | |
2330 | 109 | 23.0 | 4.4 | |
313 | 325 | 28.0 | 150 | |
109 | 36 | 4.3 | 1.8 | |
Note: Red indicates that the index exceeds the Australian Drinking Water Guidelines (ADWG), with the upper limit of copper content being 2000μg/L and the upper limit of lead content being 10μg/L. The upper limits of copper and lead in drinking water in Hong Kong are the same as those in Australia.

The longer the water stays in the water supply system, the more heavy metals will be released, and even the upper limit will be exceeded. The copper content is shown to be reduced to a satisfactory level after flushing. While there was a general decrease in lead levels, one of the samples had a significant increase in lead levels, possibly due to the segregation of lead in the copper alloy to the surface, which still required flushing. The current flushing method implemented in Hong Kong includes 2 times of water stillness (3 hours and 12 hours respectively) in each cycle, compared with only one 9-hour stillness in the above Australian study, theoretically the flushing method implemented in Hong Kong is more effective in releasing heavy metals into the water for further flushing, and the flushing effect is better if Hong Kong conducts 3 flushing cycles.
In addition, this Australian study [2] showed that the higher number of flushing, the lower the lead content. The experiment consisted of 4 sets of water supply systems, the first group with leaded fittings, and the 2nd to 4th sets with leaded faucets in addition to leaded fittings. Each set of systems will be flushed 2 times a day, each flush is 200ml, water samples are taken daily for analysis, and a total of 7 days of rinsing are carried out to understand the effect of flushing:

Figure 1: The type of faucet and the lead content in different parts of the experiment
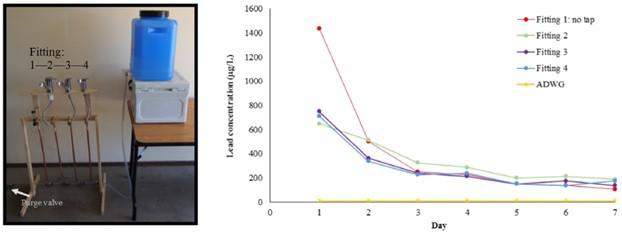
Figure 2: Experimental setup Figure 3: Changes in lead content in drinking water after flushing
The results showed that the lead content decreased significantly after 3 days of flushing, and the decrease was very small from the 4th day, and basically reached a stable state, indicating that most of the lead on the surface of the copper alloy had been released. In addition, because the copper fittings and faucets occupy a large amount of fresh water contact surface of the whole system, and the amount of flushing water (200ml) is very small, the water still contains close to 200μg/L of lead after flushing.
Systematic flushing is a quick and easy way to reduce heavy metals in new water systems without the need for any equipment or treatment systems. Some contractors are concerned that flushing may impact the construction time and expense, while plumbers are worried that they are likely to violate the law by mistake. However, in order to avoid similar incidents of excessive lead content and improve the safety of drinking water, it is necessary to implement relevant procedures. The WSD can provide more consultation and promotion, as well as the practitioners and contractors to provide feedback, to refine and enhance the implementation of the scheme.
Other suggestions:
In addition to systematic flushing and the use of pre-treated plumbing fittings or LLT fittings, the following recommendations are also effective in reducing metal content in drinking water and enhancing water safety.
- Use lead-free copper alloy fittings, please refer to NSF 372: Drinking Water System Components – Lead Content. NSF 372 is the standard method used to calculate lead levels to ensure that wet surfaces in water systems do not exceed the upper limit of 0.25% lead.
- Use a water filter that can filter out heavy metals. For example, if you use a reverse osmosis (RO) water filter, you need to ensure that it can filter the relevant heavy metals before purchasing, and you can refer to NSF 53 (Standard 53): Drinking Water Treatment Units.
- Install an automatic flushing system for drinking water system. The system automatically flushes regularly and saves the flushed water for other purposes, such as toilet flushing or household cleaning.
Reference:
[1] Guide to Application for Water Supply by WSD
[2] Wide spread copper and lead contamination of household drinking water, New South Wales, Australia, Aug. 2016, Department of Earth and Planetary Sciences, Faculty of Science and Engineering, Macquarie University, Sydney, NSW2109, Australia, Department of Environmental Sciences, Faculty of Science and Engineering, Macquarie University, Sydney, NSW2109, Australia, P.J.Harvey, H.K.Handley, M.P.Taylor




